Plasma & Monoclonal Antibody Treatments / FAQs
What antibody treatments exist?
You will hear about antibodies is relation to the following treatments:
Monoclonal antibody treatments
Direct transfusion with COVID convalescent plasma (CCP)
Hyperimmune globulin (HIG)
What are monoclonal antibodies (mAbs)?
Monoclonal antibodies (mAbs) are antibodies that are made in laboratories and help the body fight specific diseases. Treatments have received EUA and have been shown to decrease hospitalizations and improve outcomes for patients in high risk categories. They serve as substitute antibodies when the body is not making its own. They mimic the immune system’s ability to fight off harmful disease-causing agents such as the virus that causes COVID-19.
Monoclonal antibody treatment is now available around the country. It is given to newly diagnosed patients who have not been hospitalized.
The immune system detects and destroys viruses such as the one that causes COVID-19, SARS-CoV-2. One way the body's immune system attacks a virus is by making large numbers of antibodies. These antibodies stick to a specific disease-causing protein or molecule called an antigen. Once attached, antibodies can force other parts of the immune system to destroy the cells containing the antigen.
The term monoclonal antibody means that a man-made antibody is cloned and targets a specific antigen. (Polyclonal antibodies are synthesized from different immune cells and the antibodies produced bind to multiple antigens.)
Read more information from HHS.
Hay información disponible sobre anticuerpos monoclonales.
Información para médicos.
Who can receive Monoclonal Antibody (mAb) treatment?
General Patient Eligibility Requirements for Monoclonal Treatments:
POSITIVE direct viral test for SARS-CoV-2 (Antigen or PCR, rapid or non-rapid)
Within 10 DAYS of symptom onset
12 years of age or older, weighing at least 40 kg (88 pounds) who are at HIGH RISK for progressing to severe COVID-19
HIGH RISK is defined by a minimum of one of the following. Other medical conditions or factors (for example, race or ethnicity) may also place individual patients at high risk for progressing to severe COVID-19 and authorization of treatment under the EUA is not limited to the medical conditions or factors listed below. For additional information on medical conditions and factors associated with increased risk for progressing to severe COVID, see the CDC website. Healthcare providers should consider the benefit-risk for an individual patient.
· Older age (for example, age ≥65 years of age)
· Obesity or being overweight (for example, BMI >25 kg/m2, or if age 12-17, have a BMI ≥85th percentile for their age and gender based on CDC growth charts.
· Pregnancy
· Diabetes
· Immunosuppressive disease or immunosuppressive treatment
· Cardiovascular disease (including congenital heart disease) or hypertension
· Chronic lung diseases (for example, chronic obstructive pulmonary disease, asthma [moderate-to-severe], interstitial lung disease, cystic fibrosis and pulmonary hypertension)
· Sickle cell disease
· Neurodevelopmental disorders (for example, cerebral palsy) or other conditions that confer medical complexity (for example, genetic or metabolic syndromes and severe congenital anomalies)
· Having a medical-related technological dependence (for example, tracheostomy, gastrostomy, or positive pressure ventilation (not related to COVID 19))
The treatment is NOT authorized for patients:
who are hospitalized due to COVID-19, or
who require oxygen therapy due to COVID-19, or
who require an increase in baseline oxygen flow rate due to COVID-19 in those on chronic oxygen therapy due to underlying non-COVID-19 related comorbidity.
Where and how can I get treatment?
If I don’t qualify for treatment, what can I do?
If you do not qualify for treatment , consider joining a Clinical Trial.
To find out about mAb clinical trials, visit www.riseabovecovid.org, OR
Eli Lilly clinical trials: trials.lillytrialguide.com / blaze2study.com/ , OR
Regeneron clinical trials: https://www.regeneron.com/covid19.
Which Monoclonal Antibodies have been approved for Emergency Use?
On February 11th, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for a new monoclonal antibody for the treatment of COVID-19 that retains activity against the omicron variant, bebtelovimab. The EUA for bebtelovimab is for the treatment of mild to moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kilograms, which is about 88 pounds) with a positive COVID-19 test, and who are at high risk for progression to severe COVID-19, including hospitalization or death, and for whom alternative COVID-19 treatment options approved or authorized by the FDA are not accessible or clinically appropriate.
Additionally, the following anti-SARS-CoV-2 mAb products have received prior Emergency Use Authorizations (EUAs) from the Food and Drug Administration (FDA): bamlanivimab plus etesevimab, casirivimab plus imdevimab (REGEN-COV), and sotrovimab received EUAs for the treatment of mild to moderate COVID-19 in nonhospitalized patients with laboratory-confirmed SARS-CoV-2 infection who are at high risk for progressing to severe disease and/or hospitalization.
However, the distribution of bamlanivimab plus etesevimab and casirivimab plus imdevimab has been paused because the products have reduced activities against the B.1.1.529 (Omicron) variant of concern (VOC). Bebtelovimab and sotrovimab are expected to retain efficacy against the Omicron variant.
The FDA has also issued an EUA for tixagevimab plus cilgavimab (Evusheld), a long-acting anti-SARS-CoV-2 mAb combination. The EUA allows this combination to be used as SARS-CoV-2 PrEP for individuals who do not have SARS-CoV-2 infection, who have not been recently exposed to an individual with SARS-CoV-2 infection, AND who are at risk for an inadequate immune response to COVID-19 vaccination OR have a documented history of severe adverse reaction to an available COVID-19 vaccine or any of its components (see Prevention of SARS-CoV-2 Infection for more information).
From JAMA Network: “Tixagevimab plus cilgavimab is fully active against the Delta variant of SARS-CoV-2. The combination has somewhat decreased neutralizing activity in vitro against the Omicron variant (by 12- to 30-fold vs the ancestral virus); the clinical significance of this difference remains to be determined. The Omicron variant was not prevalent during clinical trials of Evusheld.”
Bebtelovimab
Bebtelovimab works by binding to the spike protein of the virus that causes COVID-19, similar to other monoclonal antibodies that have been authorized for the treatment of high-risk patients with mild to moderate COVID-19 and shown a benefit in reducing the risk of hospitalization or death.
Sotrovimab
GlaxoSmithKline’s product consists on one monoclonal antibody that is specifically directed against the spike protein of SARS-CoV-2 and is designed to block the virus’ attachment and entry into human cells. It is administered by intravenous (IV) infusion for the treatment of mild to moderate COVID-19 in adults and pediatric patients. It is recommended that this treatment be administered as soon as possible after positive viral test for SARS-CoV-2 and within 10 days of symptom onset.
Doctor Fact Sheet Patient Fact Sheet
Bamlanivimab and Etesevimab
Eli Lilly's product consists of two antibodies that target a spike protein. Emergency use has been authorized for the treatment of mild to moderate COVID-19 in adults and pediatric patients. The treatment is administered by intravenous (IV) infusion. It is recommended that this treatment be administered as soon as possible after positive viral test for SARS-CoV-2 and within 10 days of symptom onset. Eli Lilly's bamlanivimab is no longer given alone, but instead in combination with etesevimab. The combination has been shown to be more effective against variants.
More detailed information on this treatment is available here.
Doctor Fact Sheet Patient Fact Sheet.
La información en español está disponible aquí.
Casirivimab and Imdevimab
Regeneron's product consists of two monoclonal antibodies that target different spike proteins. Casirivimab and Imdevimab are administered together by injection or intravenous (IV) infusion, depending on the provider, for the treatment of mild to moderate COVID-19 in adults and pediatric patients. It is recommended that this treatment be administered as soon as possible after positive viral test for SARS-CoV-2 and within 10 days of symptom onset.
More detailed information on this treatment is available here.
Doctor Fact Sheet Patient Fact Sheet.
La información en español está disponible aquí.
Tixagevimab plus Cilgavimab (Evusheld)
Tixagevimab and cilgavimab are long-acting monoclonal antibodies that are specifically directed against the spike protein of SARS-CoV-2, designed to block the virus’ attachment and entry into human cells. Tixagevimab and cilgavimab bind to different, non-overlapping sites on the spike protein of the virus.
One dose of Evusheld, administered as two separate consecutive intramuscular injections (one injection per monoclonal antibody, given in immediate succession), may be effective for pre-exposure prevention for six months. Evusheld is not authorized for individuals for the treatment of COVID-19 or for post-exposure prevention of COVID-19. Patients should talk with their health care provider to determine whether Evusheld is an appropriate pre-exposure prevention option for them.
You can read more about the different treatments at:
Is there someone I can call to ask more questions?
Providers or patients in need of assistance locating an infusion site or connecting with a clinical trial, can call the ASPR Monoclonal Antibody Therapy Call Center. English: 877-332-6585 Spanish: 877-366-0310.
Does antibody therapy protect against viral variants?
Certain circulating SARS-CoV-2 viral variants may be associated with resistance to monoclonal antibodies. Casirivimab/Imdevimab made by Regeneron is available for use in all states. Eli Lilly’s product, Bamlanivimab/Etesevimab, is no longer being distributed to AZ, CA, FL, IL, IN, MA, OR and WA due to concerns about variant resistance. As of March 2020, bamlanivimab doses are only administered with etesevimab doses. Updated EUAs are available from the FDA.
COVID variants are evident in the USA and are tracked through genetic sequencing (state variant rates). Although sequencing information is helpful on a population level, individual patient results are not told to the provider, submitting laboratory or patient. Whole genome sequencing is not currently approved for use as a diagnostic test to make individual patient treatment decisions. In addition, the lengthy turnaround time for this type of testing means it is unsuitable for treatment decisions involving mAbs, which should be given as soon as possible once a patient has developed symptoms and tests positive. Antibody treatments, given early, remain the only option for newly diagnosed , non-hospitalized, COVID-19 patients. Looking to the future, the US governments announced in April a new 1.5 billion dollar initiative to increase the country’s ability to genetically sequence tests.
How are monoclonal antibody treatments given to a patient?
Monoclonal antibody treatment is given by injection (Regeneron only) or infusion. Since many infusion centers often treat other patients with compromised immune systems, great care must be taken by centers to ensure that patients who have COVID-19 do not come into contact with other immunocompromised patients, such as those with cancer. Infusions may be administered only in settings in which health care providers have immediate access to medications to treat a severe infusion reaction, such as allergic reactions, and the ability to activate the emergency medical system, as necessary. These requirements limit the number of locations able to provide mAbs to COVID patients.
If a hospital cannot meet these requirements, they will not offer this treatment. If a hospital receives a shipment of mAb and cannot safely distribute it, these doses will be given to another institution that can use them.
In June, treatment by injection was approved, which should increase the number of locations offering antibody treatments.
Can monoclonal antibodies be used to prevent COVID-19?
Yes, as of December 08, 2021, AstraZeneca’s product Evusheld (tixagevimab co-packaged with cilgavimab and administered together) can be used as a pre-exposure treatment for the prevention of COVID-19 in high-risk individuals. The Emergency Use Authorization (EUA) issued by the FDA for this product requires that individuals either have:
moderate to severely compromised immune systems due to a medical condition or due to taking immunosuppressive medications or treatments and may not mount an adequate immune response to COVID-19 vaccination (examples of such medical conditions or treatments can be found in the fact sheet for health care providers) or;
a history of severe adverse reactions to a COVID-19 vaccine and/or component(s) of those vaccines, therefore vaccination with an available COVID-19 vaccine, according to the approved or authorized schedule, is not recommended.
If I receive a monoclonal antibody treatment, do I still need to mask and practice social distancing?
Yes! Every day and every way. Please don't put someone else's health at risk for your own comfort.
Where can I find out more about the history of and science behind monoclonal antibodies?
For some great info, visit here and here. There’s also some great discussion at this link.
What is COVID convalescent plasma (CCP) treatment?
CCP is plasma taken from a person who has recovered from COVID-19. When you have the disease, your body creates antibodies to fight off the illness. These antibodies are in your blood plasma. When antibody-rich plasma from one person is put into another person, it can kick start their immune response and help them to recover.
Talk to your doctor about treatment with CCP. CCP is available to people who are hospitalized with COVID-19, and the latest research shows the treatment to be most effective when given as early as possible.
On September 1, 2020 the FDA issued an Emergency Use Authorization (EUA) for the use of COVID convalescent plasma as a treatment for patients hospitalized with COVID-19. Information for physicians interested in obtaining plasma for their patients is available, here. Additional information for patients and families is available, here. Your physician should work with their affiliated blood collection center or hospital to obtain plasma.
You may also gain access to convalescent plasma by enrolling in a clinical trial that will continue to track the safety and effectiveness of CCP treatments. Patients are being recruited to participate in these studies at many different medical facilities. To receive plasma treatment, you must either enter a controlled medical trial or receive permission through an Emergency Use Authorization. Trials and more information are listed here.
For more information about antibodies and plasma see our other FAQ listings.
Aprender sobre las opciones de tratamiento de covid.
What is hyperimmune globulin (HIG)?
Hyperimmune globulin (HIG) is a concentrated product manufactured from thousands of units of convalescent plasma from thousands of donors. An unprecedented partnership of industry leaders are working together to quickly and safely develop, test and produce this plasma derived treatment, which concentrates antibodies in plasma into a medicine. Equitable distribution and wide access to HIG are a priority. At this time, access to HIG is only possible through limited clinical trials. Learn more about HIG here.
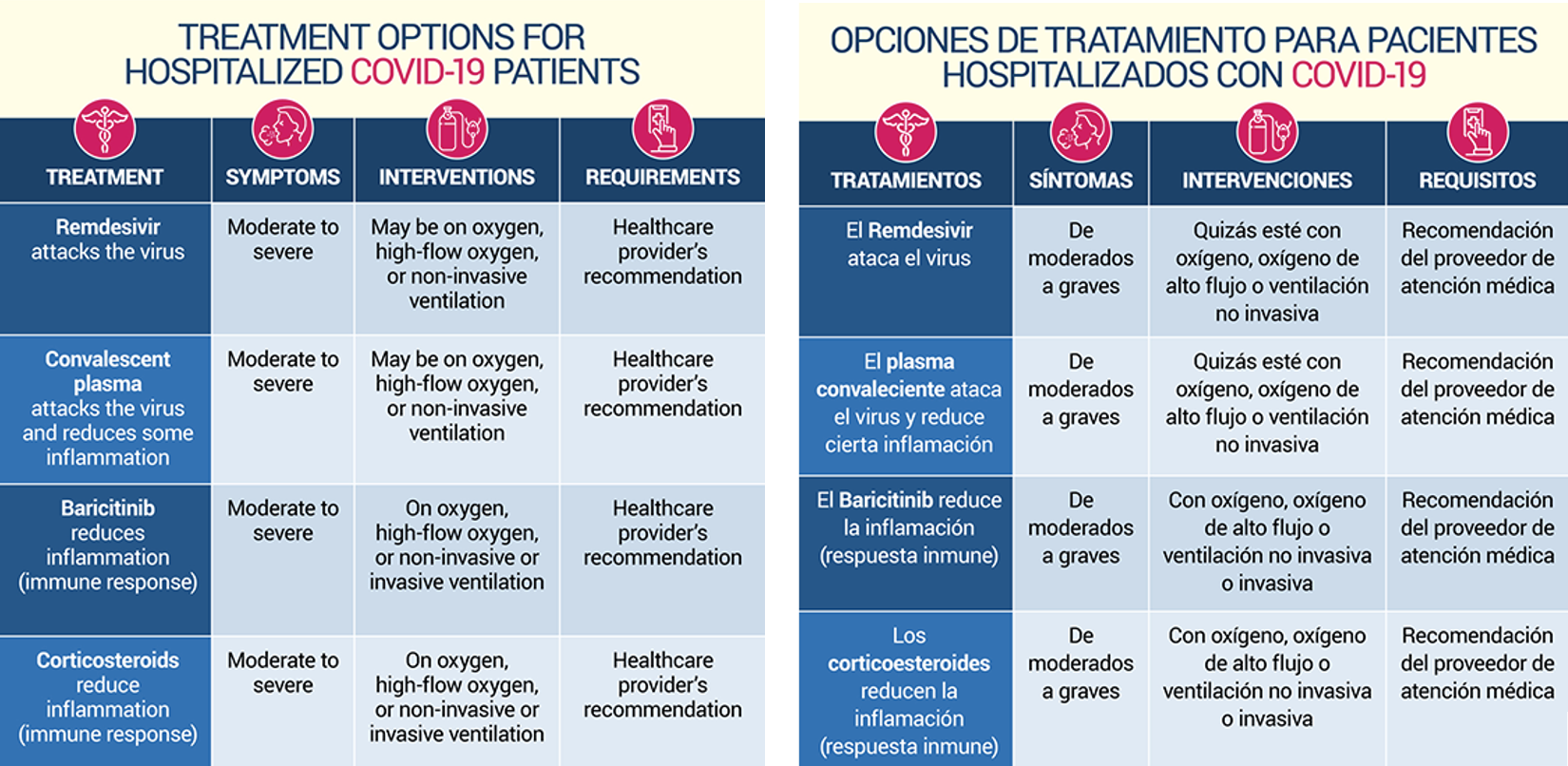
Are other treatment options available for COVID-19?
Other treatments are being used to treat COVID, and even more are being studied. At this time the treatments below are being used to treat COVID in a hospital setting or under the care of a physician. In addition the FDA has issued an Emergency Use Authorization for the use of the gammaCore Sapphire CV vagus nerve stimulation device for acute use at home or in a healthcare setting to treat adult COVID patients who are experiencing exacerbation of asthma-related dyspnea and reduced airflow, and for whom approved drug therapies are not tolerated or provide insufficient symptom relief. More information is available, here.
Additional information about treatments is available, here.